As discussed further below, scientific studies support that certain telethermographic systems, also known as thermal imaging systems, may be used to measure surface skin temperature. These systems include an infrared thermal camera and may have a temperature reference source. In this document, they are referred to as thermal imaging systems.
Thermal imaging systems and non-contact infrared thermometers (NCITs) use different forms of infrared technology to measure temperature. For information about NCITs, please refer to the fact sheet on Non-contact Infrared Thermometers.
Thermal Imaging Systems and COVID-19
- When used correctly, thermal imaging systems generally have been shown to accurately measure someone’s surface skin temperature without being physically close to the person being evaluated. Thermal imaging systems offer certain benefits in that other methods need a closer proximity or contact to measure temperature (for example, non-contact infrared thermometers or oral thermometers).
- Temperature-based screening, such as thermal imaging, is not effective at determining if someone definitively has COVID-19 because, among other things, a person with COVID-19 may not have a fever. A diagnostic test must be performed to determine if someone has COVID-19.
- Thermal imaging systems have not been shown to be accurate when used to take the temperature of multiple people at the same time. The accuracy of these systems depends on careful set-up and operation, as well as proper preparation of the person being evaluated.
- Thermal imaging systems have been used by several countries during epidemics, although information about their effectiveness as part of efforts to reduce the spread of disease has been mixed.
- The FDA issued the Enforcement Policy for Telethermographic Systems During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency guidance to help expand the availability of thermal imaging systems and mitigate thermometer shortages during the public health emergency. The guidance sets forth an enforcement policy that is intended to apply to all thermal imaging systems that are intended for medical purposes for the duration of the public health emergency related to COVID-19, and provides recommendations regarding performance and labeling of such systems.
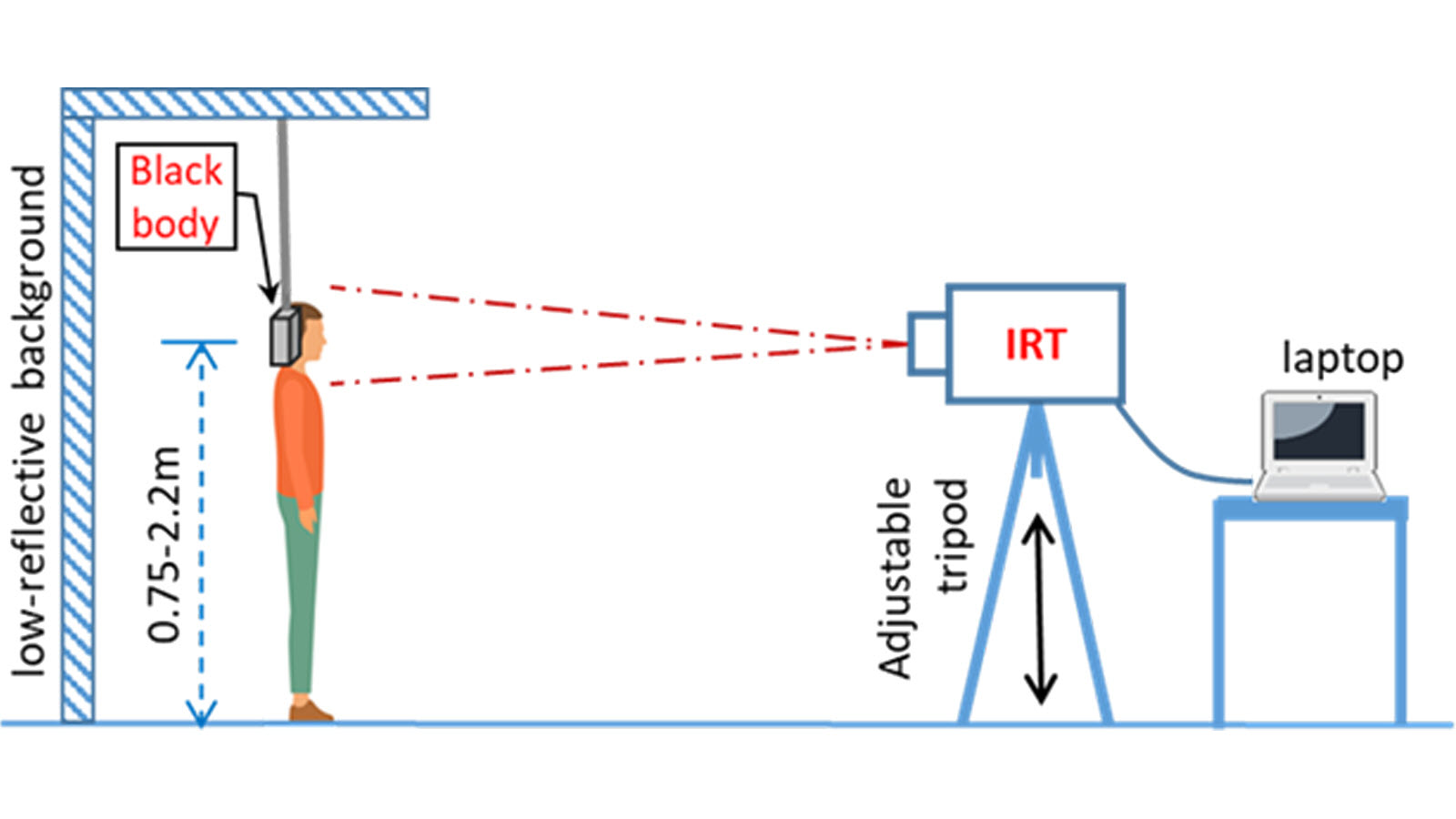
Figure 1 demonstrates the proper thermal imaging setup for processing of individual people in a public area.
Benefits of Thermal Imaging Systems
- The person who handles the thermal imaging system is not required to be physically close to the person being evaluated. In fact, the person who handles the thermal imaging system could be in a different area or room.
- The thermal imaging system may measure surface skin temperature faster than the typical forehead or oral (mouth) thermometer that requires a close distance or physical contact with the person being evaluated.
- Scientific studies show that, when used correctly, thermal imaging systems generally measure surface skin temperature accurately.
Limitations of Thermal Imaging Systems
- Although these systems may be in use for initial temperature assessment to triage individuals in high throughput areas (for example, airports, businesses and sporting events), the systems have not been shown to be effective when used to take the temperature of multiple people at the same time. They should not be used for “mass fever screening.”
- These systems measure surface skin temperature, which is usually lower than a temperature measured orally. Thermal imaging systems must be adjusted properly to correct for this difference in measurements.
- These systems work effectively only when all the following are true:
- The systems are used in the right environment or location.
- The systems are set up and operated correctly.
- The person being assessed is prepared according to instructions.
- The person handling the thermal imaging system is properly trained.
Proper Use of Thermal Imaging Systems
The person who handles the system should follow all manufacturer instructions to make sure the system is set up properly and located where it can measure surface skin temperature accurately.
The person who handles the system should be trained to properly prepare both the location where the system will be used, and the person being evaluated, to increase accuracy. For details, see the standards and scientific papers listed under References below.
Preparing the Area where You will Use a Thermal Imaging System
- Room temperature should be 68-76 °F (20-24 °C) and relative humidity 10-50 percent.
- Try to control other items that could impact the temperature measurement:
- Avoid reflective backgrounds (for example, glass, mirrors, metallic surfaces) to minimize reflected infrared radiation.
- Use in a room with no draft (movement of air), out of direct sunlight and away from radiant heat (for example, portable heaters, electrical sources).
- Avoid strong lighting (for example, incandescent, halogen and quartz tungsten halogen light bulbs).
Figure 2 demonstrates the proper thermal imaging room setup.
Preparing the Thermal Imaging System
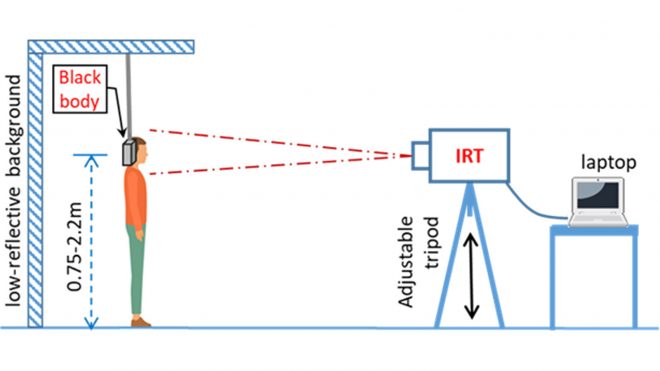
- Some systems require the use of a calibrated blackbody (a tool for checking the calibration of an infrared temperature sensor) during evaluation to make sure measurements are accurate. Check the manufacturer’s instructions to determine if a calibrated blackbody is needed. Some devices do not require one.
- Turn on the entire system 30 minutes before use to warm it up.
Preparing the Person Being Evaluated
The person handling the system should make sure the person being evaluated:
- Does not have any face obstructions before measurement (such as a mask, glasses, hat, headband, or scarf), the person’s hair is pulled away from the face, and the person’s face is clean and dry.
- Does not have a higher or lower face temperature from wearing excessive clothing or head covers (for example, headbands, bandanas) or from using facial cleansing products (for example, cosmetic wipes).
- Has waited at least 15 minutes in the measurement room or 30 minutes after exercising, strenuous physical activity, bathing, or using hot or cold compresses on the face.
Figure 3 demonstrates the proper thermal imaging setup for processing of individual people using a calibrated blackbody background.
Using the Thermal Imaging System
- Measure only one person’s surface skin temperature at a time.
- Position the person at a fixed distance (follow the manufacturer’s instructions for use) from the thermal imaging system, directly facing the camera.
- The image area should include the person’s whole face and the calibrated blackbody, if using one.
- If an increased temperature is seen using the thermal imaging system, you should use a different method to confirm a fever. Public health officials can help you determine if the fever is a sign of infection.
Questions about Using Thermal Imaging Systems during COVID-19
Q: Are thermal imaging systems effective for screening people for fevers in places like nursing homes, airports, and hospital emergency rooms?
A: When using a thermal imaging system, it is important to assess whether the system will provide the intended results in high throughput areas. We understand that these devices are being used for initial temperature assessment and triage of individuals for elevated temperatures in medical and non-medical environments. They should not be used for measuring temperatures of many people at the same time in crowded areas, in other words “mass fever screening” is not recommended.
Based on where the system will be used, there may be more appropriate methods to initially assess and triage people, especially if there is a risk that infected people would not be identified right away. For example:
- In a nursing home, inaccurate temperature measurement or a missed contagious person without a fever could spread infection among nursing home residents. So, in this case, other assessment options and following infection control practices may be more effective.
- In airports, workplaces, grocery stores, concert venues, or other areas where you are trying to screen large groups of people for mass fever screening, diagnostic testing may be too difficult because of the time and costs needed to screen and get results. These systems will likely miss most individuals with COVID-19 who are contagious. Thermal imaging systems could be considered as one method for initial temperature assessment in these types of settings when used as part of a larger approach to risk management.
- In a hospital emergency room, a thermal imaging system may help to quickly assess temperature and triage patients to determine who needs more evaluation or isolation.
Q: Are thermal imaging systems effective as the sole means of diagnosing COVID-19?
A: No. A fever or higher body temperature is only one possible symptom of a COVID-19 infection. Thermal imaging systems generally detect a high body temperature accurately when used appropriately. They do not detect any other infection symptoms, and many people with COVID-19 can be contagious without a fever. Also, a high body temperature does not necessarily mean a person has a COVID-19 infection.
All fevers measured by thermal imaging systems should be confirmed by another method and followed by more diagnostic evaluations for other symptoms, as appropriate.
Q: How can thermal imaging systems help with the COVID-19 response?
A: To help address urgent public health concerns raised by shortages of temperature measurement products and expand the availability of telethermographic systems used for initial body temperature for triage use during this COVID-19 public health emergency, the FDA is applying regulatory flexibility for certain telethermographic systems as outlined in its enforcement policy.
When a high body temperature is identified by thermal imaging, an additional evaluation should follow (for example, doctor evaluations or interview, laboratory testing and patient observation).
Q: Are thermal imaging systems used for body temperature assessment considered medical devices?
A: As discussed in the enforcement policy, telethermographic systems are devices when they are intended for a medical purpose. To determine if these products are intended for a medical purpose, FDA will consider whether:
- They are labeled or otherwise intended for use by a health care professional;
- They are labeled or otherwise for use in a health care facility or environment; and
- They are labeled for an intended use that meets the definition of a device, for example, body temperature measurement for diagnostic purposes, including in non-medical environments.
Q: How does a thermal imaging system differ from a thermometer?
A: Both thermal imaging systems and non-contact infrared thermometers (NCIT) can measure surface temperatures without contact. An NCIT measures surface temperature in a single location, whereas a thermal imaging system can measure temperature differences across multiple locations, creating a relative temperature map of a region of the body. The enforcement policy in the guidance applies to use of thermal imaging systems to determine initial body temperature measurements.
There is a separate enforcement policy that applies to certain NCITs and other clinical electronic thermometers: Enforcement Policy for Clinical Electronic Thermometers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency.
References
Note, this information is applicable to thermal imaging systems that are intended for a medical purpose. This means that the system is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease and, therefore, meets the definition of “device” set forth in Section 201(h) of the Federal Food, Drug, and Cosmetic Act.
For more information on FDA’s policies for these devices, and recommendations on their design, labeling, and use during the COVID-19 Public Health Emergency, please review the following:
Additional information on these devices can be found at:
IEC 80601-2-59: Medical electrical equipment – Part 2-59: Particular requirements for basic safety and essential performance of screening thermoghraphs for human febrile temperature screening. 2017, International Electrotechnical Commission & International Organization for Standardization.
ISO/TR 13154: Medical electrical equipment — Deployment, implementation and operational guidelines for identifying febrile humans using a screening thermograph. 2017, International Organization for Standardization.
Ghassemi, P., et al. (2018). “Best practices for standardized performance testing of infrared thermographs intended for fever screening.” PLoS ONE 13(9): e0203302External Link Disclaimer.
Comparing Temperature Sensor Devices
- Industrial infrared temperature sensors are inexpensive and used everywhere in home and industrial. Your microwave for example. They read surface temperature if aimed properly and clean and calibrated (all sensors require cleaning and calibration)
- The Heimann sensor is the first we encountered. The HPTA32x32 (64 pixel) “thermopile array”.
- Melexis makes several models (all TO-39)
- Mitsubishi, Elo and many others make these.
- They have been adapted for reading temperatures of foreheads basically and are at the low end of the accuracy scale.
- None of these are FDA approved or submitted.
- Thermal Imaging Cameras
- These are a whole magnitude higher weight-class. Several of them are specifically designed for supplemental elevated body temperature. They do this by zooming and focusing on specific areas of the face like tearducts. IR Arrays have limited spatial resolution and must average many regions and samples in order to provide a general value.
- FLIR is the manufacturer most used. They have several which certified by FDA and others submitted.
- Another option is ICI though there are some questions on FDA 510 (we cannot locate them) as well as some components used.
Temperature Related More Reading