Kiosk Manufacturer Association Temperature Kiosk Public Service Announcement
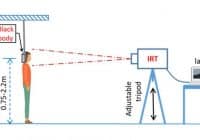
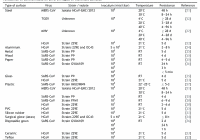
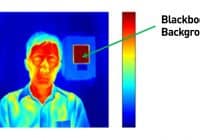
From PRNewswire Jul2020 — The Kiosk Manufacturer Association (KMA) is issuing a cautionary advisory regarding temperature check kiosks and fever detection kiosks. DENVER, July 6, 2020 /PRNewswire/ — As government and corporations across the world develop post-COVID-19 action plans for responsibly reopening, some businesses are scrambling to keep up with the demand for thermal cameras, which many believe can help… Read More »