Temperature Screening Kiosk KMA Technical Advisory
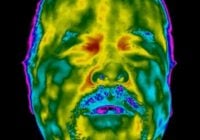
From KMA Global Jun2020 Temperature Kiosks Advisory and Caution – Public Service Announcement By KMA Manager | June 29, 2020 As government and corporate America develop post-COVID-19 action plans for responsibly reopening the country, some businesses are scrambling to keep up with the demand for thermal cameras, which many believe can help identify novel coronavirus cases via elevated temperature detection. We… Read More »